|
Mercaptan:
any of a class of organosulfur compounds is similar to the
alcohol and phenol but containing a sulfur atom in place of the oxygen atom.
Compounds containing -SH as the principal group directly attached to carbon are
named 'thiols'. In substitutive nomenclature their names are formed by adding
'-thiol' as a suffix to the name of the parent compound. When -SH is not the
principal group, the prefix 'mercapto-' is placed before the name of the parent
compound to denote an unsubstituted -SH group. 'thio' is a chemical prefix
indicates the replacement of an oxygen in an acid radical by sulfur with a
negative valence of 2. Sulfur analog of alcohol is called thiol (or mercaptan), and ether analog
is called sulfide.
The first chemical contrast of thiols and sulfides with
alcohols and ethers is acidity which is important in organic reactions. Thiols
are stronger acids than relevant alcohols and phenols.Thiolate conjugate bases
are easily formed, and are excellent nucleophiles in SN2 reactions of alkyl
halides and tosylates. The nucleophilicity of sulfur is much greater than that
of oxygen, resulting in a number of useful electrophilic substitution reaction
that are rare by oxygen. For example, sulfides form (with alkyl halides) ternary
sulfonium salts, in the same alkylattion of tert-amines quaternary ammonium
salts, whereas ternary oxonium salts are prepared only under extream conditions.
Without exception, sulfoxides, sulfinate salts and sulfite anion also alkylate
on sulfur, despite of the partial negative formal charge on oxygen and partial
positive charge on sulfur. The second character is the oxidation states of
sulfur. Oxygen has only two oxidation states, whereas sulfur covers from –2 to
+6 as follows:
- -2: Hydrogen Sulfide (H2S), sulfides, sulfonium ions
- -1:
disulfides
- 0: S elemental, sulfoxides, sulfenic acids
- +2: sulfones,
sulfinic acids
- +4: sulfonic acids, sulfite esters
- +6: sulfate
esters
One more sulfur compound's contrast with oxygen analog is in oxidation
chemistry. Oxidation of sulfur compounds changes the oxidation state of sulfur
rather than carbon, whereas, oxidation of alcohols to aldehydes and ketones
changes the oxidation state of carbon not oxygen. Thiols is oxidized to S-S
single bond (disufide) which is stronger than O–O bond in peroxide. Disufide
forms sulfenyl chlorides (with chlorine in mild condition) or sulfonic acids
under harder condition. Oxidation of sulfides with hydrogen peroxide (or
peracids) yields sulfoxides and then to sulfones. A certain sulfoxide compound
such as dimethyl sulfoxide can be used as an effective oxygen source in the
oxidation reaction of primary and secondary alcohols to aldehydes and ketones.
DMSO easily is reduced to dimethyl sulfide and water is taken up by the
electrophile. oxidation procedure is very mild and tolerates a variety of other
functional groups, including those having oxidizable nitrogen and sulfur
atoms.
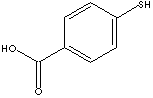
p-Mercaptobenzoic acid
is used as an intermediate
for
manufacturing synthetic organic compounds,
pharmaceuticals, colorants and agrochemicals.
|
Benzoic acid,
the simplest aromatic carboxylic acid containing carboxyl group bonded directly
to benzene ring, is a white, crystalline organic compound; melting at 122 C
(starting sublime at 100 C); boiling at 249 C; slightly soluble in water,
soluble in ethanol, very slightly soluble in benzene and acetone. Its aqua
solution is weakly acidic. It occurs naturally in many plants and resins.
Benzoic acid is also detected in animals. The most of commercial benzoic acid is
produced by the reaction of toluene with oxygen at temperatures around 200 C in
the liquid phase and in the presence of cobalt and manganese salts as catalysts.
It can be prepared also by the oxidation of benzene with concentrated sulphuric
acid or carbon dioxide in the presence of catalysts. Other methods are such as
by the oxidation of benzyl alcohol, benzaldehyde, cinnamic acid; by hydrolysis
of benzonitrile, benzoyl chloride. More than 90% of commercial benzoic acid is
converted directly to phenol and caprolactam. Its use in the production of
glycol benzoates for the application of plasticizer in adhesive formulations is
increasing. It is also used in the manufacture of alkyd resins and drilling mud
additive for crude oil recovery applications. It is used as a rubber
polymerization activators and retardants. Benzoic acid is converted to its salts
and esters for the use of preservative application in foods, drugs and personal
products. Sodium benzoate, sodium salt of benzoic acid, is used preferably as
one of the principal anti-microbial preservatives used in foods and beverages
(but it's concentration is limited usually not exceeding 0.1% because it is
poisonous), as it is about 200 times more soluble than benzoic acid. Sodium
Benzoate is also used in medications, anti-fermentation additives and tabletting
lubricant for pharmaceuticals. The industrial applications are as a corrosion
inhibitor, as an additive to automotive engine antifreeze coolants and in other
waterborne systems, as a nucleating agents for polyolefin, as a dye
intermediate, as a stabilizer in photographic processing and as a catalyst. Wide
range of benzoic esters are used as solvents, dying carrier, disinfectant
additive, penetrating agent and pesticides and manufacturing other compounds.
|